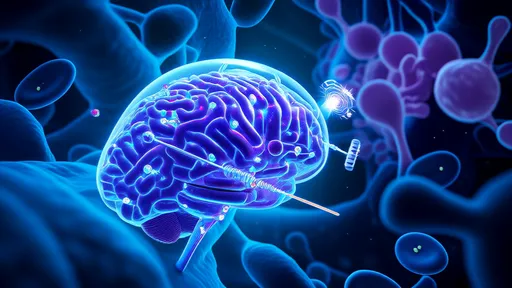
In a groundbreaking development that could redefine aging research, scientists have unveiled a novel approach to clearing senescent cells—dubbed the "cellular incinerator" mechanism. This technique leverages enhanced lysosomal autophagy to selectively target and eliminate these dysfunctional cells, which accumulate with age and contribute to tissue decline. The discovery, spearheaded by a team at the Aging Research Institute of Basel, offers a promising avenue for interventions aimed at extending healthspan.
The "Incinerator" Mechanism: A Paradigm Shift
Senescent cells, long implicated in age-related diseases, resist apoptosis and secrete inflammatory factors that disrupt tissue function. Traditional senolytic drugs force these cells into apoptosis, but the new approach takes a different tack. By hyperactivating lysosomes—the cell's waste-disposal units—researchers observed a dramatic uptick in the engulfment and degradation of senescent cells. "It's like flipping a switch to turn senescent cells into self-destructing waste," explains Dr. Elena Vogt, lead author of the study published in Nature Cell Biology. The process, termed LYSA (Lysosomal Senescent-cell Autophagy), appears to bypass the inflammatory side effects associated with conventional senolytics.
Lysosomes: From Garbage Collectors to Executioners
What makes this discovery remarkable is its exploitation of lysosomal activity beyond routine cellular cleanup. The team identified a cluster of proteins, including the previously obscure TMEM175 channel, that act as molecular triggers. When stimulated, these proteins cause lysosomes to expand dramatically, engulfing entire senescent cells—organelles and all—before digesting them. Intriguingly, healthy cells remain unaffected due to their tighter regulation of lysosomal pH. "This selectivity is nature’s gift," notes Dr. Vogt. "We’re essentially weaponizing a natural process that already distinguishes between healthy and senescent cells."
Animal Trials Show Striking Results
In aged mice, LYSA activation reduced senescent cell burden by 70% within eight weeks—far surpassing the 30-50% clearance rates of pharmaceutical senolytics. Treated animals exhibited improved muscle function, reduced frailty, and even regrowth of hair follicles. Perhaps most compelling was the absence of toxicity; unlike drugs that stress kidneys or liver, LYSA relies on endogenous pathways. "The mice didn’t just live longer—they vaulted through obstacle courses like adolescents," remarks Dr. Simon Karr, a biogerontologist unaffiliated with the study. "This isn’t merely delaying aging; it’s reversing aspects of it."
The Human Horizon: Challenges and Promise
Translating LYSA to humans presents hurdles. Lysosomal activity varies widely between individuals, and overactivation risks triggering pathologies like lysosomal storage disorders. The team is developing precision modulators to fine-tune the process. Early-stage human cell cultures respond similarly to murine models, but clinical trials remain years away. Still, the implications are profound: if successful, LYSA could transform aging from a passive process to a modifiable condition. As Dr. Vogt puts it: "We’re not just adding years to life—we’re adding life to years."
Ethical and Commercial Landscape
The technology has already attracted venture capital, with startups racing to patent lysosomal modulators. Yet ethical questions loom. Should LYSA be classified as therapy or enhancement? Could it exacerbate socioeconomic disparities? Meanwhile, Big Pharma watches closely—many hold lucrative patents on traditional senolytics that LYSA may render obsolete. The coming decade will test whether science can navigate these complexities to deliver what might be humanity’s most radical intervention against aging yet.
By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025

By /Jul 29, 2025
By /Jul 29, 2025

By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025