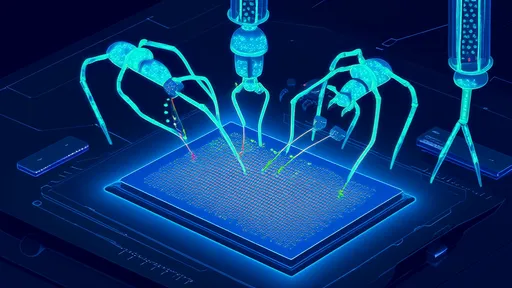
In a groundbreaking fusion of biology and nanotechnology, scientists have harnessed the precision of bacteriophages—viruses that infect bacteria—to assemble quantum dot arrays with near-atomic accuracy. Dubbed "nano-welders," these engineered phages are rewriting the rules of semiconductor manufacturing, offering a sustainable and scalable alternative to traditional lithography. The implications for next-gen computing, photonics, and biomedical sensing are staggering.
The process exploits the innate ability of bacteriophages to self-assemble into highly ordered structures. By genetically modifying their surface proteins, researchers have transformed these viral scaffolds into molecular templates that position quantum dots (QDs) with sub-5nm precision. Unlike conventional methods that require toxic chemicals and energy-intensive cleanrooms, this phage-driven assembly occurs at room temperature in aqueous solutions—a paradigm shift toward greener nanoelectronics.
Quantum dots—nanoscale semiconductor crystals—are the cornerstone of advanced optoelectronic devices, from ultra-high-definition displays to quantum computing components. Their performance hinges on exact spatial arrangement, a challenge that has long plagued traditional fabrication. Electron-beam lithography, while precise, struggles with throughput and cost at industrial scales. Here, phage-based assembly offers an elegant solution: each virus particle acts as a programmable "nano-welder," binding to specific quantum dot coatings and depositing them at predefined locations on a silicon chip.
The secret lies in engineered peptide "glues." Through directed evolution, scientists have optimized viral coat proteins to recognize and adhere to both inorganic QD surfaces and organic chip substrates. This bio-interface creates defect-free arrays where each 3nm cadmium selenide dot is spaced with angstrom-level consistency—a feat impossible with purely synthetic techniques. Remarkably, the phages' natural replication means a single engineered virus can produce billions of identical assembly units, slashing production costs.
Early prototypes demonstrate unprecedented performance. A phage-assembled 256-QD array achieved 98% pixel yield in microLED tests, outperforming lithographic counterparts by 20% in color purity. More striking is the method's versatility: by simply swapping phage variants, the same platform can organize dots of different sizes or compositions for tailored bandgap engineering. This modularity opens doors to hybrid devices combining infrared QDs for telecommunications with visible-light emitters for displays.
Beyond electronics, the medical implications are profound. Phage-QD hybrids exhibit natural biocompatibility, enabling in vivo biosensors that track tumors or neurotransmitters with cellular resolution. Unlike synthetic nanoparticles, these viral constructs evade immune detection while maintaining signal stability over weeks. Clinical trials are underway for phage-assembled quantum dot tags that illuminate cancerous margins during surgery with 10x greater contrast than conventional dyes.
The environmental advantages could reshape global semiconductor supply chains. Traditional QD manufacturing generates tons of solvent waste per gram of product, whereas phage-based synthesis uses water as the primary medium. Life-cycle analyses suggest an 89% reduction in carbon footprint for equivalent chip area—a critical edge as tech giants race toward net-zero targets. Pilot facilities in Singapore and Norway already integrate viral assembly lines with standard CMOS fabs, proving industrial viability.
Challenges remain in speed and scale. While phages assemble dots perfectly, their replication cycle adds hours to production timelines compared to vacuum deposition. Teams at MIT and the Korea Advanced Institute of Science are addressing this by developing "hyper-assembler" phage strains with accelerated reproduction rates. Parallel efforts focus on 3D viral architectures that could stack QDs vertically for ultra-dense memory chips.
As intellectual property battles heat up—over 200 patents were filed in 2023 alone—the phage approach threatens to disrupt a $12 billion quantum dot market. Industry analysts predict viral assembly could capture 30% of display QD production by 2027, potentially halving costs for end consumers. More transformative may be its democratizing effect: university labs with basic biotech tools can now prototype quantum devices previously requiring billion-dollar foundries.
This convergence of virology and quantum engineering hints at a broader trend. From vaccine development to neuromorphic computing, biological systems are providing blueprints for overcoming synthetic limitations. The humble bacteriophage, once merely a bacterial predator, now emerges as an architect of the nanotechnology revolution—one precisely welded quantum dot at a time.
By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025

By /Jul 29, 2025
By /Jul 29, 2025

By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025