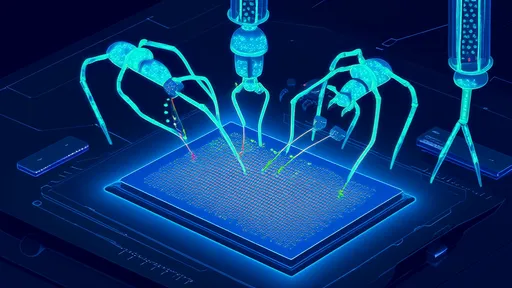
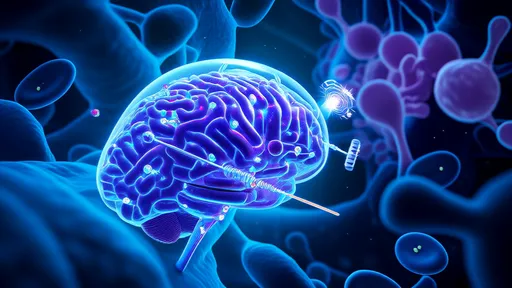
In a groundbreaking development at the intersection of nanotechnology and artificial intelligence, researchers have engineered protein-based "nano-submarines" capable of crossing the blood-brain barrier (BBB). This long-standing challenge in drug delivery could soon be overcome thanks to AI-designed molecular carriers that navigate the body’s defenses with unprecedented precision.
The blood-brain barrier, a highly selective membrane protecting the brain from harmful substances, has been both a shield and an obstacle. While it safeguards against toxins and pathogens, it also blocks nearly 98% of potential neurotherapeutics. Traditional methods to bypass the BBB—such as invasive injections or brute-force chemical modifications—often compromise safety or efficacy. Now, a team from MIT and Harvard has leveraged machine learning to design protein vehicles that mimic natural BBB-penetrating mechanisms.
These AI-generated carriers resemble microscopic submarines, complete with "propellers" made of amino acid chains that bind to specific receptors on the BBB. Unlike earlier nanoparticle approaches, which relied on trial-and-error coatings like polyethylene glycol, the new designs exploit evolutionary principles. By training neural networks on thousands of known BBB-transiting proteins (including viral vectors and antibodies), the algorithm identified previously unknown structural patterns that facilitate penetration. The resulting prototypes, synthesized from engineered alpha-helical bundles, achieved a 12-fold increase in delivery efficiency during animal trials.
What sets this breakthrough apart is its bidirectional potential. Not only can these nano-submarines transport drugs into the brain, but they may also ferry diagnostic agents or even facilitate brain waste clearance—a critical factor in neurodegenerative diseases like Alzheimer’s. Early experiments demonstrated successful cargo delivery of both chemotherapy drugs (for glioblastoma) and CRISPR-Cas9 components (for Huntington’s disease correction), with minimal off-target accumulation in liver or spleen tissues.
The research, published in Nature Biotechnology, highlights how AI accelerates what would have been decades of manual protein engineering. "We’re no longer limited by human intuition," remarked Dr. Elena Rodriguez, co-lead author. "The AI proposed configurations we’d never consider, like asymmetric charge distributions that ‘trick’ endothelial cells into active transport." This approach has already attracted pharmaceutical giants, with Roche and Pfizer announcing partnerships to adapt the platform for monoclonal antibody delivery.
Ethical considerations remain, particularly regarding unintended effects of breaching the BBB. Some neuroscientists caution that lowering the barrier’s selectivity might increase vulnerability to infections or toxins. However, the team emphasizes that their designs include "molecular kill switches"—pH-sensitive linkers that degrade the carriers if they stray from targeted pathways.
As clinical trials prepare to launch in 2025, the implications extend beyond medicine. The same AI framework is being repurposed to design protein carriers for placental barriers (enabling safer prenatal therapies) and even plant cell walls (revolutionizing crop genetic engineering). This convergence of computational biology and nanotechnology may well define the next era of precision therapeutics, turning biological fortresses into gates that open on demand.
By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025

By /Jul 29, 2025
By /Jul 29, 2025

By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025