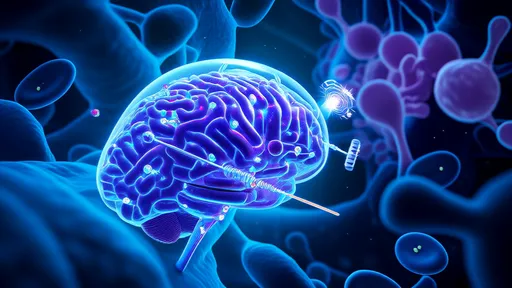
In a groundbreaking development that could revolutionize cellular medicine, researchers have pioneered a novel approach to mitochondrial therapy using engineered exosomes as biological delivery vehicles. Dubbed "mitochondrial power banks," these customized nanoscale carriers show remarkable potential for treating diseases linked to impaired cellular energy production.
The science builds upon a simple yet profound concept: when mitochondria malfunction, cells lose their power supply. Traditional gene therapies often struggle to address these defects, as mitochondrial DNA operates independently from nuclear DNA. This innovation bypasses genetic complexity by delivering readymade healthy mitochondria directly into compromised cells.
Exosomes: Nature's Perfect Delivery System
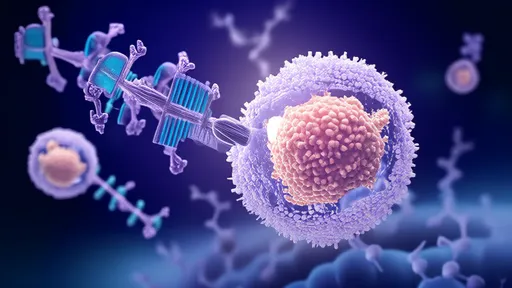
Exosomes, the body's natural intercellular messengers, have emerged as ideal carriers for mitochondrial transfer. These lipid-bound vesicles already shuttle biological materials between cells without triggering immune responses. Scientists now harvest exosomes from mesenchymal stem cells, loading them with functional mitochondria through a proprietary electroporation process.
"What makes exosomes extraordinary is their biological camouflage," explains Dr. Elena Vasquez, lead researcher at the MITO Therapeutics Institute. "They're like microscopic Trojan horses that slip past cellular defenses. Our engineering process preserves this stealth capability while equipping them with therapeutic cargo."
The Engineering Breakthrough
Creating mitochondrial-loaded exosomes required solving two critical challenges: maintaining mitochondrial viability during encapsulation and ensuring proper release upon reaching target cells. The team developed a gentle centrifugation protocol that preserves mitochondrial membrane potential while packaging them into exosomes.
Advanced imaging reveals how the engineered exosomes fuse with recipient cell membranes, depositing their mitochondrial payload directly into the cytoplasm. Unlike previous methods involving whole-cell transplantation, this approach minimizes rejection risks while achieving superior mitochondrial integration rates.
Clinical Applications Emerge
Early-stage trials demonstrate particular promise for neurodegenerative conditions. In Parkinson's disease models, mitochondrial exosomes crossed the blood-brain barrier and restored dopaminergic neuron function. Cardiologists also report successful myocardial uptake in ischemic heart tissue, potentially offering new hope for post-heart attack recovery.
Dr. Rajiv Patel's team at Stanford observed unexpected benefits: "Beyond energy restoration, we're seeing mitochondrial transfer modulate inflammatory responses. This dual-action effect could transform treatment paradigms for autoimmune disorders."
Manufacturing Challenges
Scaling production remains the technology's primary hurdle. Current protocols yield limited quantities of therapeutic-grade mitochondrial exosomes. Several biotech firms are racing to develop bioreactor systems that can mass-produce these complex biologics while maintaining strict quality control.
Regulatory pathways also require navigation. As neither pure drug nor cell therapy, mitochondrial exosomes inhabit a unique classification space. The FDA recently established a new review committee specifically for mitochondrial transfer products, signaling recognition of the field's potential.
Future Horizons
Researchers envision applications extending beyond disease treatment. Athletic performance enhancement and anti-aging interventions show preclinical promise. A controversial but active research branch explores combining mitochondrial exosomes with CRISPR technology for targeted organ rejuvenation.
Investment has surged, with venture capital funding exceeding $300 million in 2023 alone. Pharmaceutical giants are acquiring startups in the space, though many scientists caution that widespread clinical use remains years away.
As the science progresses, ethical questions emerge regarding enhancement versus therapy. The same technology that might cure muscular dystrophy could theoretically boost athletes' endurance. Such dual-use potential ensures mitochondrial exosomes will remain both medically exciting and socially consequential.
The coming years will determine whether this "cellular power bank" approach fulfills its transformative potential. For now, it stands as a shining example of bioengineering ingenuity - harnessing nature's own delivery systems to heal at humanity's most fundamental biological level.
By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025

By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025

By /Jul 29, 2025
By /Jul 29, 2025

By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025
By /Jul 29, 2025